Neuronal secretory pathway
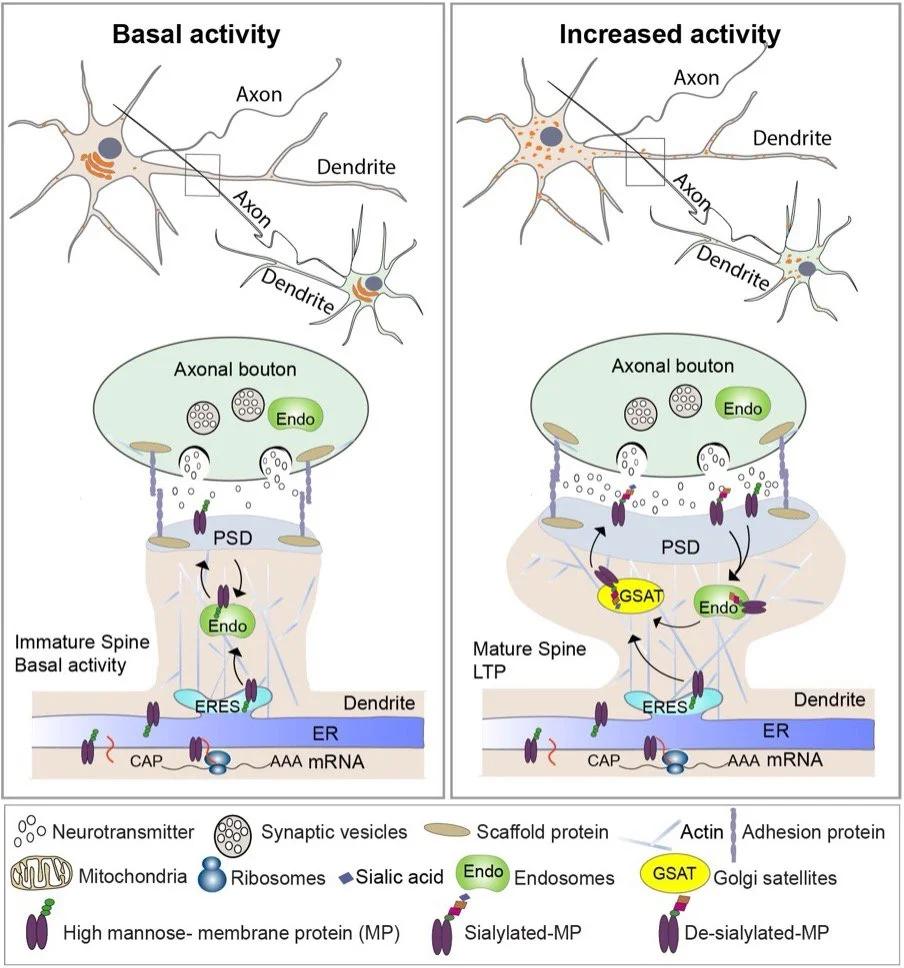
Model of activity-induced glycosylation at synapses mediated by Golgi satellites
A major focus of the lab is to understand how drugs of abuse induce long-lasting changes at synapses that remodel neural circuits and contribute to addiction. We have recently uncovered novel effects of nicotine and synaptic activity on the organization of the neuronal secretory pathway. In neurons, nicotine exposure or membrane excitation disrupts the compact, perinuclear Golgi structure, leading to the formation of scattered Golgi elements positioned near ER exit sites and endosomes (see model). These structures, known as Golgi satellites, are mobile within dendrites and axons and become more abundant in response to neuronal activity or nicotine treatment. In vivo nicotine exposure also increases Golgi satellite formation in dopaminergic axon terminals of neurons in the midbrain ventral tegmental area (VTA), a key region in the brain’s reward circuitry.
We have identified several cargo proteins, including the α4β2-subtype nicotinic acetylcholine receptor (α4β2R), that traffic through Golgi satellites and undergo dynamic glycosylation. This is particularly significant because prolonged nicotine exposure is known to increase high-affinity α4β2R binding sites in the brain, a process termed “upregulation” that is associated with nicotine craving and withdrawal.
Current projects use rodent cellular models and advanced imaging techniques to define the signaling pathways that regulate Golgi satellite formation and function. These studies have important implications for understanding neuronal cell biology, elucidating nicotine’s addictive properties, and informing the development of improved smoking cessation therapies. We are also exploring whether other forms of neuronal activity or synaptic plasticity similarly influence Golgi satellite morphology, trafficking, and function.
Golgi satellite mobility in the axon of a nicotine-stimulated neuron
Golgi satellite mobility in the axon of an unstimulated neuron